Search Results
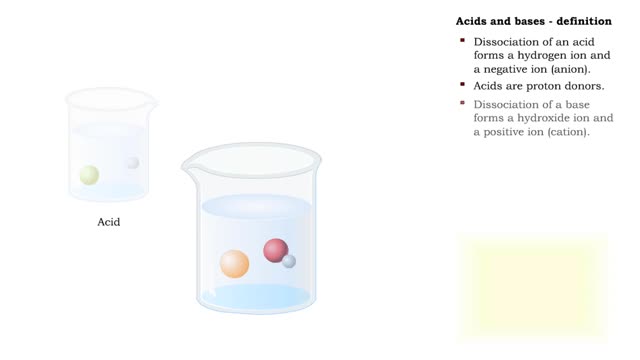
Results for: 'is a weak acid. • Few molecules dissociate because a strong attraction exists between H and HCO5. درجة الحموضة: ھو مقیاس لتحدید تركیز أیونات الھیدروجین H في المحلول یُرمز لدرجة الحموضة بالرمز (pH) وتُعرف أیضاً بالرقم الھیدروجیني أو الأس الهيدروجيني . درجة الحموضة مقیاس مدّرج من 0 إلى 14 ویُعبّر عن تركیز أیونات H وأیونات -OH في المحلول. فالمحالیل الحمضیة تمتلك قیمة pH أقل من (7)، وكلما قلت قیمة pH للحمض زادت قوتھ. والمحالیل القاعدیة تمتلك قیمة pH أكبر من (7)، وكلما زادت قیمة pH للقاعدة زادت قوتھا. أما الماء المقطر فتبلغ قیمة pH لھ (7)، أي أنھ یُعتبر متعادلاً وفق ھذا المقیاس لأن تركیز أیونات-OH مساٍو لتركیز أیونات H تعتمد قيمة PH للمحلول على تركيز كل من أيون الهيدروجين الموجب H وأيون الهيدروكسيد السالب OH-'
The pH scale - Strong acids and Weak acids
By: HWC, Views: 6715
The pH scale • Expresses concentration of H+. • range: 0-14. • 7 is neutral. • Less 7 is acid. • greater 7 is basic (alkaline). Strong acids - role in the body ■ In strong acids all molecules dissociate. ■ HC1 is highly acidic and found only in the stomach. • H...
Buffers definition and the role of buffer in the body
By: HWC, Views: 6804
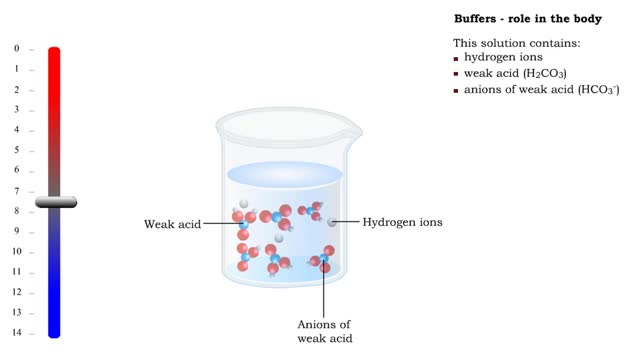
■ Too many H+ break hydrogen bonds and a protein comes apart. ■ Buffers react with excess H+ to protect proteins from breaking down. ■ Buffers consist of weak acid plus anions of that weak acid. This solution contains: • hydrogen ions • weak acid (H2CO3) • anions of we...
Ionic bonds - role of ions in the body
By: HWC, Views: 6875
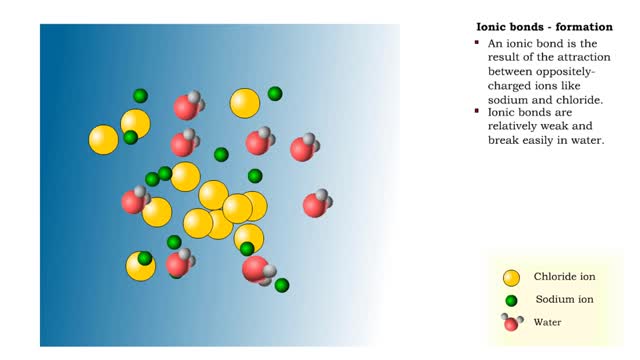
Ions • Atoms fill up the outer orbital by transferring electrons from one atom to another. • Atoms now bear a charge and are called ions. • Sodium ion, losing an electron, has a +1 charge. • Chlorine ion, gaining an electron, has a -1 charge. Formation • An ionic bond is t...
What are Strong & Weak Acids and How they're different?
By: HWC, Views: 5485
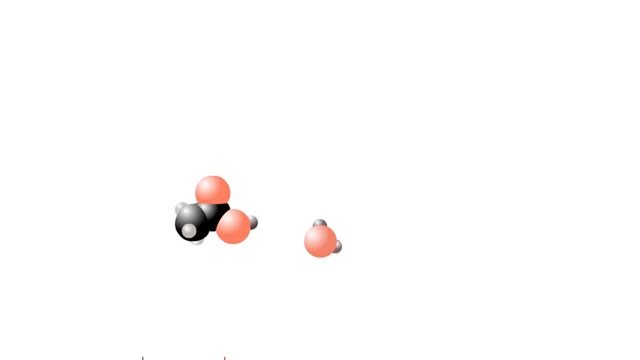
Let's consider the changes that take place when hydrogen chloride, HCI, is added to water. You will need to recognize space-filling models of HCI molecules, hydronium ions (H30+), chloride ions (C11, and water molecules (H20). They are shown at the right. When HC1 molecules dissolve in water, ...
By: HWC, Views: 7023
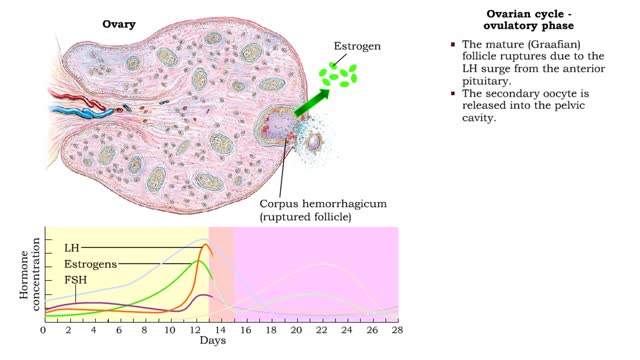
• The ovarian cycle is a monthly sequence of events, consisting of three phases: • Preovulatory • Ovulatory • Post ovulatory Preovulatory phase • prior to ovulation: Primary follicles develop into secondary follicles. • Follicular cells surrounding the primary oocyte In...
Hydrogen bonds - role in the body
By: HWC, Views: 7013
A hydrogen bond is the electromagnetic attraction between polar molecules in which hydrogen is bound to a larger atom, such as oxygen or nitrogen. This is not a sharing of electrons, as in a covalent bond. Instead, this is an attraction between the positive and negative poles of charged atoms. ...
Krebs cycle : Formation of acetyl coenzyme A and Electron transport chain
By: HWC, Views: 6779
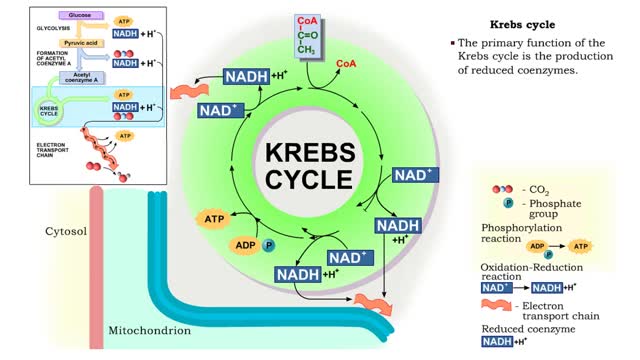
The oxidation of glucose to produce ATP is cellular respiration. Four sets of reactions are involved: Glycolysis Formation of acetyl coenzyme A Krebs cycle reactions Electron transport chain reactions • The second pathway of glucose catabolism, formation of acetyl coenzyme A, is a transi...
Glucose anabolism reactions: Glycogenolysis and Gluconeogenesis
By: HWC, Views: 7003
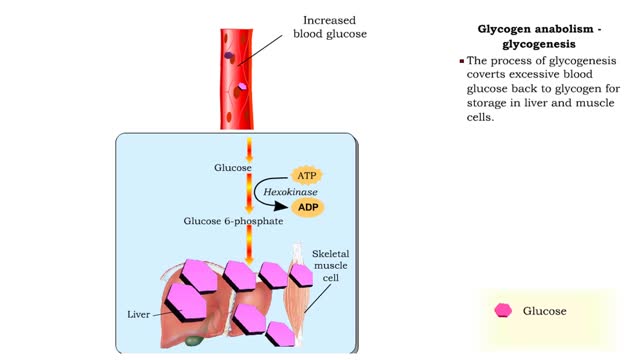
• Glucose not needed immediately is stored as glycogen. The process that creates it is glycogenesis. • When ATP is needed for body activities, stored glycogen is broken down by a process called glycogenolysis. • Glucose can be formed through two different anabolic reactions: • Glycog...
By: HWC, Views: 6592
Acids and bases are found all around your house. For example, if you open your pantry or refrigerator, you might see a lot of acids. Fruit juice, soda pop, vinegar, and milk are all examples of acids. The word acid actually comes from a Latin term meaning ''sour.'' Many materials, like sugar for ...
Advertisement